Johnson Matthey and Alfa Aesar support new platinum group metals research
Polyoxometalates (POMs) are a large class of discrete, soluble metal-oxo anions of early transition metals in high oxidation states, such as tungsten(VI) or molybdenum(VI). Due to a unique combination of properties, such as thermal and oxidative stability, tunability of acidity and redox activity, solubility in various media, and ability to undergo multistep multi-electron transfers without structural changes, POMs keep attracting more and more attention in different areas of fundamental and industrial science, in particular in homogeneous and heterogeneous catalysis.
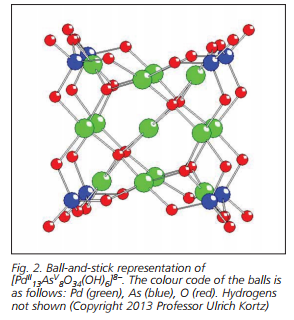
Kortz’s group are world leaders in the synthesis and characterisation of noble metal-containing polyanions. They prepared the first example of a Pt(IV)-containing polyoxovanadate, [H2PtIVV9O28]5- by a facile synthetic procedure, using the Pt(IV) precursor H2Pt(OH)6. The polyanion [H2PtIVV9O28]5- was characterised in the solid state by X-ray diffraction (XRD) and in solution by 195Pt and 51V NMR spectroscopy.
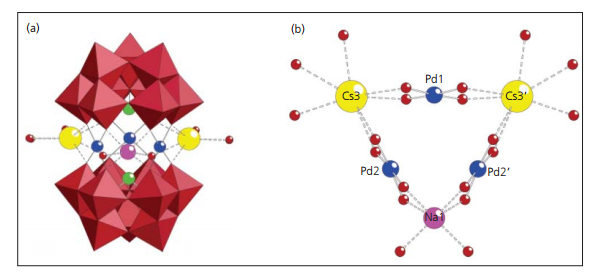
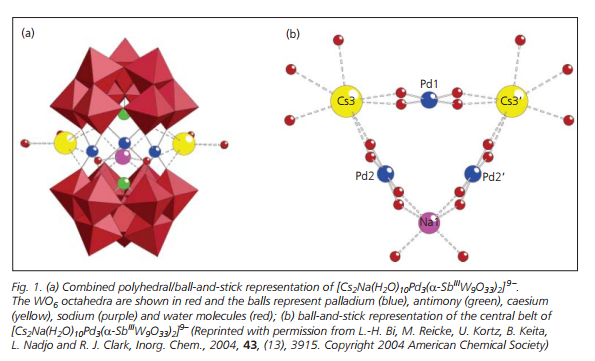
Their research also includes the first example of a Pd(II)-containing heteropolyoxometalate, [Cs2Na(H2O)10Pd3(α-SbIIIW9O33)2]9- which consists of two (α-SbW9O33) moieties linked by three square planar-coordinate Pd2+ ions resulting in a sandwich type structure (Figure 1(a)). The central belt is completed by two Cs+ and a Na+ ion which occupy the vacancies between the adjacent Pd centres, resulting in a polyanion with idealised C2v symmetry (Figure 1(b)).
Selected Publications
- Y. Xiang, N. V. Izarova, F. Schinle, O. Hampe, B. Keita and U. Kortz, Chem. Commun., 2012, 48, (79), 9849 LINK http://dx.doi.org/10.1039/c2cc33795a
- M. Barsukova-Stuckart, N. V. Izarova, R. A. Barrett, Z. Wang, J. van Tol, H. W. Kroto, N. S. Dalal, P. Jiménez-Lozano, J. J. Carbó, J. M. Poblet, M. S. von Gernler, T. Drewello, P. de Oliveira, B. Keita and U. Kortz, Inorg. Chem., 2012, 51, (24), 13214 LINK http://dx.doi.org/10.1021/ic301537n
- N. V. Izarova, M. T. Pope and U. Kortz, Angew. Chem. Int. Ed., 2012, 51, (38), 9492 LINK http://dx.doi.org/10.1002/anie.201202750
- N. V. Izarova, A. Banerjee and U. Kortz, Inorg. Chem., 2011, 50, (20), 10379 LINK http://dx.doi.org/10.1021/ic201451x
- N. V. Izarova, N. Vankova, T. Heine, R. N. Biboum, B. Keita, L. Nadjo and U. Kortz, Angew. Chem. Int. Ed., 2010, 49, (10), 1886 LINK http://dx.doi.org/10.1002/anie.200905566 N. V. Izarova, N. Vankova, T. Heine, R. N. Biboum, B. Keita, L. Nadjo and U. Kortz, Angew. Chem. Int. Ed., 2010, 49, (10), 1886 LINK http://dx.doi.org/10.1002/anie.200905566
- N. V. Izarova, N. Vankova, A. Banerjee, G. B. Jameson, T. Heine, F. Schinle, O. Hampe and U. Kortz, Angew. Chem. Int. Ed., 2010, 49, 7807 LINK http://dx.doi.org/10.1002/anie.201001738
- E. V. Chubarova and U. Kortz, Exxonmobil Chemical Company, ‘Novel Heteropolyanions with Late Transition Metal Addenda Atoms and Process for their Preparation’, US Patent Appl. 2009/0,216,052
- U. Lee, H.-C. Joo, K.-M. Park, S. S. Mal, U. Kortz, B. Keita and L. Nadjo, Angew. Chem. Int. Ed., 2008, 47, (4), 793 LINK http://dx.doi.org/10.1002/anie.200703082
- E. V. Chubarova, M. H. Dickman, B. Keita, L. Nadjo, F. Miserque, M. Mifsud, I. W. C. E. Arends and U. Kortz, Angew. Chem. Int. Ed., 2008, 47, (49), 9542 LINK http://dx.doi.org/10.1002/anie.200803527
- L.-H. Bi, M. Reicke, U. Kortz, B. Keita, L. Nadjo and R. J. Clark, Inorg. Chem., 2004, 43, (13), 3915 LINK http://dx.doi.org/10.1021/ic049736d